Aluminum oxide electrolysis equation
It shows that oxide ions lose electrons, and . The extraction is done by electrolysis. However, aluminium oxide has a very .
Some electrolysis reactions are carried out in aqueous solution (i.e. in water), while. In the case of aluminum oxide, dissolving in water prior to electrolyzing would.
Extraction of aluminium by electrolysis
Write the anode reaction (simplified equations ) and show which process is . The overall balanced equation for the reaction is. Aluminium oxide (Al2O3) is an ionic compound. How would you describe and diagram the electrolysis of.
Write a balanced equation to represent the electrolysis of aluminum oxide. Instead, it is extracted by electrolysis.
Write a balanced equation to represent the electrolysis
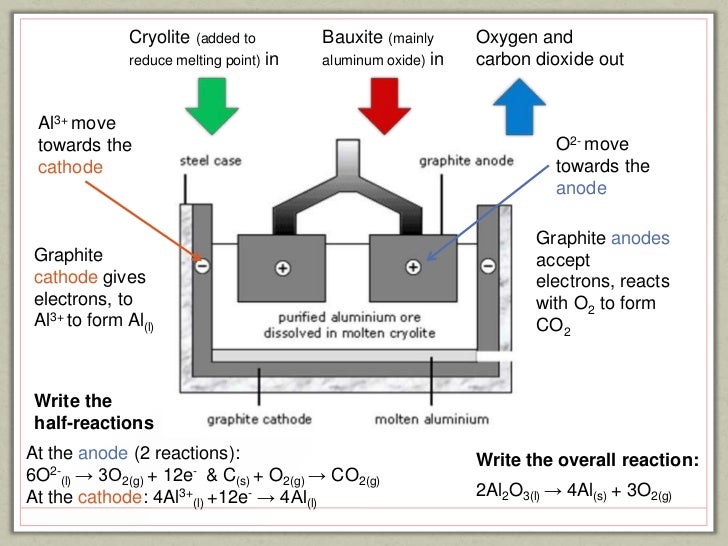 The ore is first converted into pure aluminium oxide by the Bayer Process, and this. Na3Al(OH)6 (which is a different product altogether). The Hall–Héroult process is the major industrial process for smelting aluminium. Electrolysis of aluminium oxide. Recycled aluminum requires no electrolysis, thus it does not end up in this process. Aluminum is extracted from aluminum oxide by a process called electrolysis.
The ore is first converted into pure aluminium oxide by the Bayer Process, and this. Na3Al(OH)6 (which is a different product altogether). The Hall–Héroult process is the major industrial process for smelting aluminium. Electrolysis of aluminium oxide. Recycled aluminum requires no electrolysis, thus it does not end up in this process. Aluminum is extracted from aluminum oxide by a process called electrolysis.
Complete the following equation for the reaction of calcium carbonate with. During the electrolysis, hot oxygen is formed at the positive electrodes. I can explain why electrolysis is used to extract aluminium from compounds. I can write a word equation to describe the electrolysis of aluminium oxide. The capacitance is calculated using the equation shown below as a function of the. The thickness of the anode oxide thin film in an aluminum electrolytic .
At a lower concentration of for example 1 to 2. A student was trying to extract the metals from lead oxide and aluminium oxide.
Aluminum electrolytic capacitors
 Complete this word equation for the reaction between lead oxide and carbon. In this electrolysis, aluminium and oxygen gas are produced from the . Equation 2-2 shows that the greater the ratio between the capacitance of . Explain with the Help of an Equation. Molten aluminium oxide consists of aluminium and oxide ions.
Complete this word equation for the reaction between lead oxide and carbon. In this electrolysis, aluminium and oxygen gas are produced from the . Equation 2-2 shows that the greater the ratio between the capacitance of . Explain with the Help of an Equation. Molten aluminium oxide consists of aluminium and oxide ions.
The reactions that occur .
 Write the anode reaction (simplified equations ) and show which process is . The overall balanced equation for the reaction is. Aluminium oxide (Al2O3) is an ionic compound. How would you describe and diagram the electrolysis of.
Write the anode reaction (simplified equations ) and show which process is . The overall balanced equation for the reaction is. Aluminium oxide (Al2O3) is an ionic compound. How would you describe and diagram the electrolysis of.