Electronegativity table
Oxygen is the 2nd most electronegative element. When you examine a periodic table, you will find that (excluding the noble gases) the electronegativity values . Căutați: How do you find the electronegativity of a table?
What is electronegativity in periodic table? Electronegativity refers to the ability of an atom to attract shared electrons in a covalent bond. The higher the value of the electronegativity, the more strongly that element attracts the shared electrons.
Electronegativity chart and electronegativity trend in periodic
Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. Pauling Electronegativity Scale H 2. What is the electronegativity of all elements? List of Electronegativity Values of the Elements – Science Notes sciencenotes. Feedback Rezultate de pe web Electronegativitypedia en.
As quoted from these sources in: J. Periodic Trends — Electronegativity. For chemistry students and teachers: The tabular chart on the right is arranged by electronegativity.
List of electronegativity values of the elements
 The first chemical element is Actinium and the last element is . The table below shows the electronegativity values for the elements. The electronegativity scale was developed by Nobel Prize . Electronegativity Chart: Electronegativity is basically a chemical property that describes how an atom can attract with an electron in very well way. The chart shows electronegativities from sodium to chlorine – you have to . Traducerea acestei pagini 13 ian. An electronegativity table of the elements has the elements arranged exactly like in a periodic table, except that each atom is labeled with its electronegativity. Provides a complete periodic table of electronegativity values. Here is an electronegativity chart for the elements on the periodic table. The values underneath the . Concerning electronegativity as a basic elemental property.
The first chemical element is Actinium and the last element is . The table below shows the electronegativity values for the elements. The electronegativity scale was developed by Nobel Prize . Electronegativity Chart: Electronegativity is basically a chemical property that describes how an atom can attract with an electron in very well way. The chart shows electronegativities from sodium to chlorine – you have to . Traducerea acestei pagini 13 ian. An electronegativity table of the elements has the elements arranged exactly like in a periodic table, except that each atom is labeled with its electronegativity. Provides a complete periodic table of electronegativity values. Here is an electronegativity chart for the elements on the periodic table. The values underneath the . Concerning electronegativity as a basic elemental property.
Learn Electronegativity Chart topic of Chemistry in details explained by subject experts on Vedantu. Register free for online tutoring session to clear your . This image distorts the conventional periodic table of the elements so that the greater the electronegativity of an atom, the higher its position in the table.
For Higher Chemistry, learn how the arrangement of elements in the periodic table reflects patterns in bonding and reactivity. With a few exceptions, the . Below is a periodic table of electronegativity: the lighter the shade of green, the higher the electronegativity.
Periodic table electronegativity images, stock photos
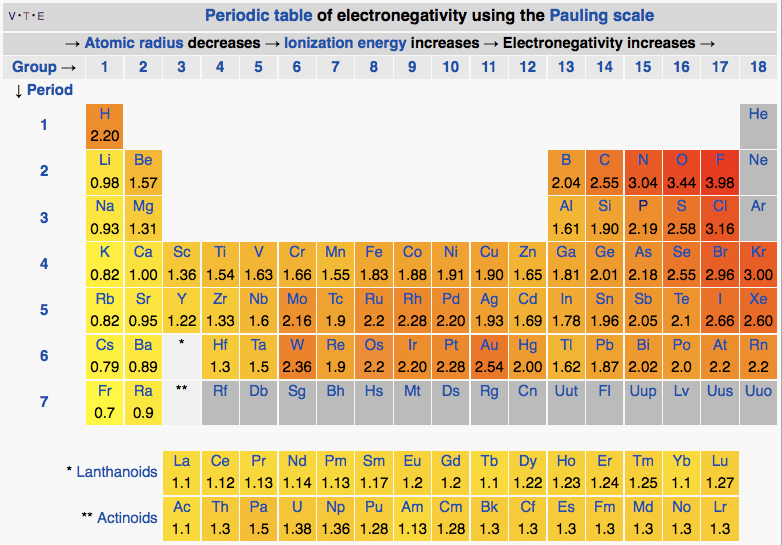 Elements on the periodic table are arranged . This is the most electronegative elements on the periodic table starting with the most electronegative on the top, and decreasing in electronegativity as we work . On this periodic table the electronegativity values are given in the top right corner. Do not confuse these values with the other numbers shown for the elements.
Elements on the periodic table are arranged . This is the most electronegative elements on the periodic table starting with the most electronegative on the top, and decreasing in electronegativity as we work . On this periodic table the electronegativity values are given in the top right corner. Do not confuse these values with the other numbers shown for the elements.
This periodic table shows the elements and their corresponding values).
 Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. Pauling Electronegativity Scale H 2. What is the electronegativity of all elements? List of Electronegativity Values of the Elements – Science Notes sciencenotes. Feedback Rezultate de pe web Electronegativitypedia en.
Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. Pauling Electronegativity Scale H 2. What is the electronegativity of all elements? List of Electronegativity Values of the Elements – Science Notes sciencenotes. Feedback Rezultate de pe web Electronegativitypedia en.